Biological Molecules
Ingredients of life
Bibliography: William Prout
- Became fascinated with human digestion, especially the urine. He thought the best way to understand human body is through chemistry, and the best way to understand the body’s chemistry was to understand what it does to food.
- In the morning before coming to the hospital as a doctor, he did some great things that led to discovery of hydrochloric acid in stomachs and a breakthrough book about kidney stones called “An inquiry into the Nature and Treatment of gravel, calculus, and other diseases connected with a deranged operation of the urinary organs”.
- The first person who discovered the chemical composition of pure urea.
- Came to the conclusion that all “foodstuffs” fell into three categories: the saccharonous (carbohydrates), oleaginous (fats), and albuminous (proteins).
1. Carbohydrates
- Sources of energy that we have available to us.
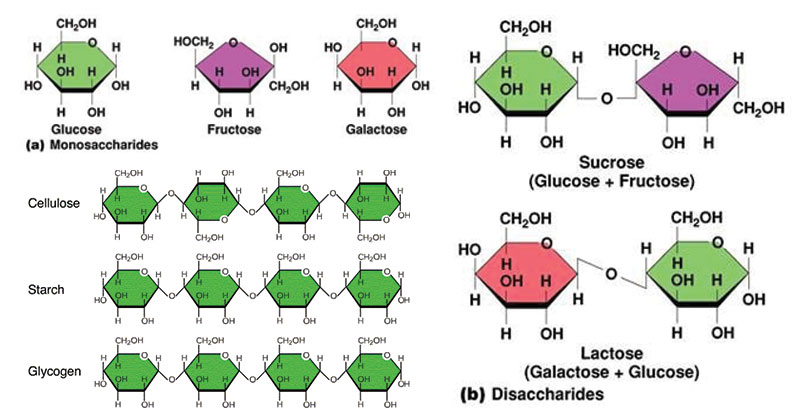
- Made up of sugars, the simplest of them are monosaccharides. Monosaccharides and disaccharides are easy for the body to process so they are the instant sources of energy.
- Monocchacarides: glucose (synthesized by the plants from photosynthesis), fructose.
- Disaccharides: sucrose (a glucose and fructose joined by covalent bond).
- Larger saccharides are harder to be digested so they become storehouse of energy or structural compounds.Polysaccharides can contain thousands of sugar units.
- Cellulose –> human can’t digest.
- Starch: amylose –> human can digest.
- Glycogen: basically made up of the glucose we have left over after we eat and it seats in our muscles where it’s ready to be used and it’s also stored in our livers.
 (Image source: https://microbenotes.com/carbohydrates/)
(Image source: https://microbenotes.com/carbohydrates/)
2. Lipids
- Smaller and simpler than complex carbonhydrates, and they group together because they share an inability to dissolve in water.
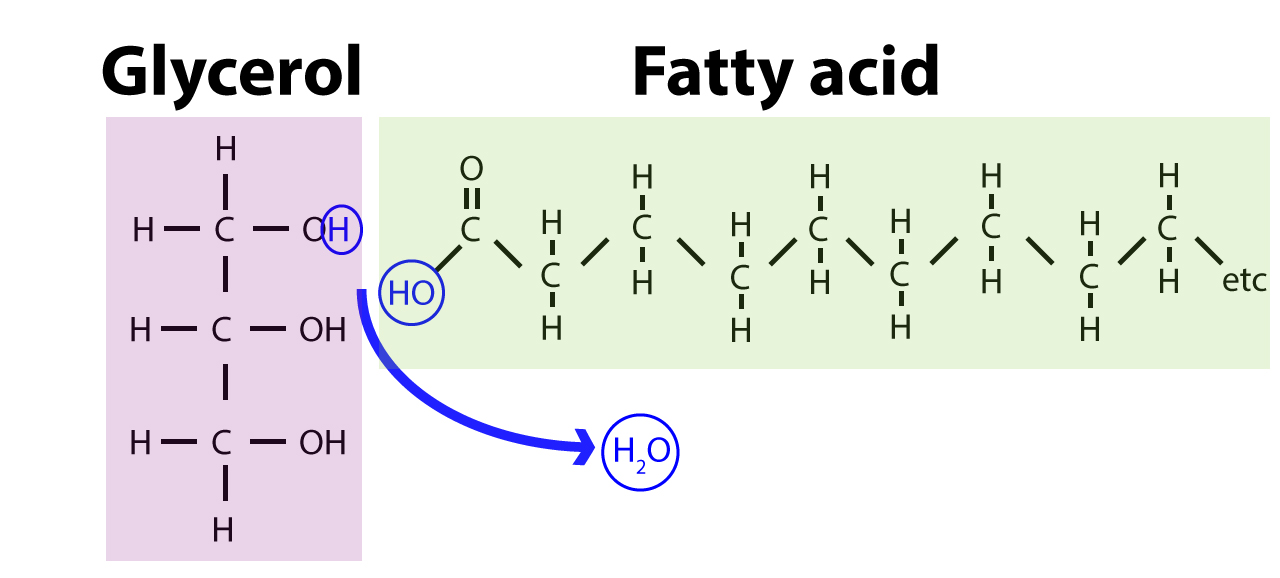
- Made up mainly of two chemical ingredients: glycerol (a kind of alcohol) and fatty acid, which are long carbon-hydrogen chains that end in a carboxyl group.
 (Image source: https://www.visionlearning.com/es/library/Biologia/2/Lipids/207)
(Image source: https://www.visionlearning.com/es/library/Biologia/2/Lipids/207) - When three fatty acid molecules connect to a glycerol, we get a triglyceride. Triglyceride can be saturated (carbon atoms are connected with each other by single bond) or unsaturated (carbon atoms are connected with each other with double bonds).
- Trans-fats are not good for human’s health. Omega-3 fatty acids are unsaturated at the 3 position, humans can’t synthesize them and they are essential fatty acids, meaning that we need to eat them.
- Swab one of 3 fatty acids in triglyceride for a phosphate group, we will have a phospholipid. These make up cell membrane wall.
- one end polarity, which is attracted to water.
- another end is nonpolar and it avoids water. –> When we put a bunch of phospholipids into some water, they would automatically arrange themselves with hydrophobic ends facing each other, and hydrophilic end sticking out to face the water.
- Steroids: have a backbone of 4 interconnected carbon rings, which can be used to form hundreds of variations. The most fundamental of them is cholesterol, which binds with phospholipids to help form cell walls. They can also activated into lipid hormones: estradiol, testosterone.
3. Proteins
- The most complicated compound in the body.
- Enzymes, antibodies, protein hormones.
- Use 20 ingredients - amino acids (amino group + carboxyl group + R group). 9 amino acid that we can made by ourselves: Histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threnonine, tryptophan, and valine.
- Amino acids connect to each other forming polypeptides.